1 Answer
From Chem WIKI:
Chemistry of Ozone Depletion
CFC molecules are made up of chlorine, fluorine and carbon atoms and are extremely stable. This extreme stability allows CFC's to slowly make their way into the stratosphere (most molecules decompose before they can cross into the stratosphere from the troposphere). This prolonged life in the atmosphere allows them to reach great altitudes where photons are more energetic. When the CFC's come into contact with these high energy photons, their individual components are freed from the whole. The following reaction displays how Cl atoms have an ozone destroying cycle:
Chlorine is able to destroy so much of the ozone because it acts as a catalyst. Chlorine initiates the breakdown of ozone and combines with a freed oxygen to create two oxygen molecules. After each reaction, chlorine begins the destructive cycle again with another ozone molecule. One chlorine atom can thereby destroy thousands of ozone molecules. Because ozone molecules are being broken down they are unable to absorb any ultraviolet light so we experience more intense UV radiation at the earths surface.
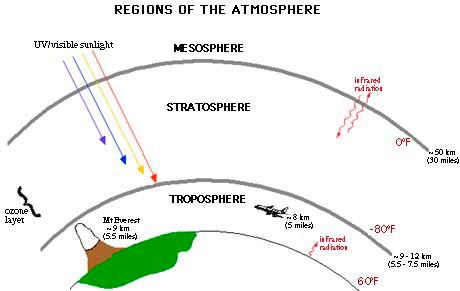
Figure 2. Much like sunscreen for the Earth, the ozone layer shields the Earth from the sun’s damaging UV-B radiation, which can adversely affect human health and ecosystems. Figure courtesy of NOAA.
| 10 years ago. Rating: 2 | |

 Jody holder
Jody holder




